Abstract
Background: With advances in oral oncolytics for hematologic malignancies, it is important to consider dosing frequency and treatment (tx) adherence, as prior data have shown poor adherence to oral cancer medications may result in inferior tx outcomes (Huang WC, Expert Rev Anticancer Ther, 2016; Muluneh B, J Oncol Pract, 2018). There is limited evidence regarding the impact of dosing frequency on tx adherence among patients with hematological malignancies treated with oral oncolytics. The objective of this study was to characterize real-world demographic and clinical characteristics, and compare refill adherence, among patients with hematological malignancies receiving once-daily (QD) versus twice-daily (BID) oral oncolytics.
Methods: This retrospective cohort study used multi-year (2012-2020) commercial claims data from Optum's de-identified Clinformatics ® Data Mart Database and included adults (≥18 yr) with a diagnosis of a hematological malignancy identified using ICD-9-CM and ICD-10-CM codes. The exposure of interest was receipt of an oral oncolytic, and the date of the first observed oral oncolytic (QD or BID) was considered the index date. All patients were required to have continuous health plan enrollment for ≥6 mo prior and ≥6 mo after the index date. The 6-mo period prior to the index date was considered the baseline period for determining patient demographics, clinical characteristics, and prior medication use. To minimize the effect of the differences in observable baseline patient characteristics between the QD and BID groups on refill adherence, 1:1 propensity score (PS) matching was used. Tx refill adherence to QD and BID oral oncolytics was measured using two established metrics: proportion of days covered (PDC) and medication possession ratio (MPR). PDC, a conservative measure of refill adherence, was considered for primary analysis as it measures total days of drug coverage within a pre-specified follow-up period; whereas MPR which was included for sensitivity analysis, measures total drug supplied within the pre-specified follow-up period. PDC and MPR were measured at pre-defined time periods of 3 and 6 mo, and from the index date until the end of the follow-up period (variable-length follow-up). Patients with drug coverage for ≥80% (PDC or MPR ≥0.8) of pre-defined time periods were considered tx refill adherent.
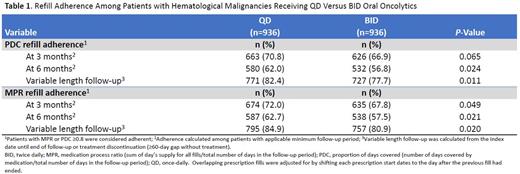
Results: The study identified 5,874 adults with hematological malignancies, of whom 4,938 (84.1%) received QD oral oncolytics and 936 (15.9%) received BID oral oncolytics. Before 1:1 PS matching, there were significant differences between the two groups in terms of mean age (67.5 yr QD vs 61.6 yr BID, P<0.001), insurance type (Medicare, 65.4% QD vs 46.8% BID, P<0.001), being previously untreated (67.6% QD vs 63.7% BID, P=0.019), and Charlson's comorbidity score (mean: 1.61 QD vs 1.44 BID, P<0.001). After 1:1 PS matching, demographics, baseline comorbidities, cancer histology, and line of therapy were well-balanced. The matched cohort comprised 936 patients receiving QD oral oncolytics and 936 patients receiving BID oral oncolytics. Table 1 summarizes refill adherence among patients with hematological malignancies receiving QD versus BID oral oncolytics. The proportion of patients with ≥80% of drug coverage during follow-up was higher for QD versus BID recipients at 3 mo: numerically higher by PDC (70.8% vs 66.9%, P=0.065) and statistically significantly higher by MPR (72% vs 67.8%, P=0.049). The proportion of adherent patients was significantly higher for QD vs BID for both PDC and MPR at 6 mo (PDC: 62% vs 56.8%, P=0.024; MPR: 62.7% vs 57.5%, P=0.021) and at variable-length follow-up (PDC: 82.4% QD vs 77.7% BID, P=0.011; MPR: 84.9% QD vs 80.9%, P=0.020). These results suggest QD dosing is associated with improved tx adherence to oral hematological oncolytics compared to BID dosing.
Conclusions: This study demonstrates significantly higher refill adherence in patients receiving QD versus BID oral hematological oncolytics at 6 mo and variable-length follow-up, highlighting the potential benefit of dosing convenience in improving adherence to oral oncolytics in the real-world setting. As suboptimal tx adherence may result in reduced tx effectiveness, real-world characterization of refill adherence rates of oral oncolytics can better inform on tx effectiveness outside of a controlled clinical trial environment.
Challagulla: Pharmacyclics LLC, an AbbVie Company: Current Employment; AbbVie: Current equity holder in publicly-traded company. Kebede: Pharmacyclics LLC, an AbbVie Company: Consultancy; ObsEva: Other. Rege: Pharmacyclics LLC, an AbbVie Company (paid to institution): Consultancy. Volodarsky: AbbVie: Current equity holder in publicly-traded company; Pharmacyclics LLC, an AbbVie Company: Current Employment. Osei-Bonsu: AbbVie: Current equity holder in publicly-traded company; Pharmacyclics LLC, an AbbVie Company: Current Employment. Karve: AbbVie: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal